

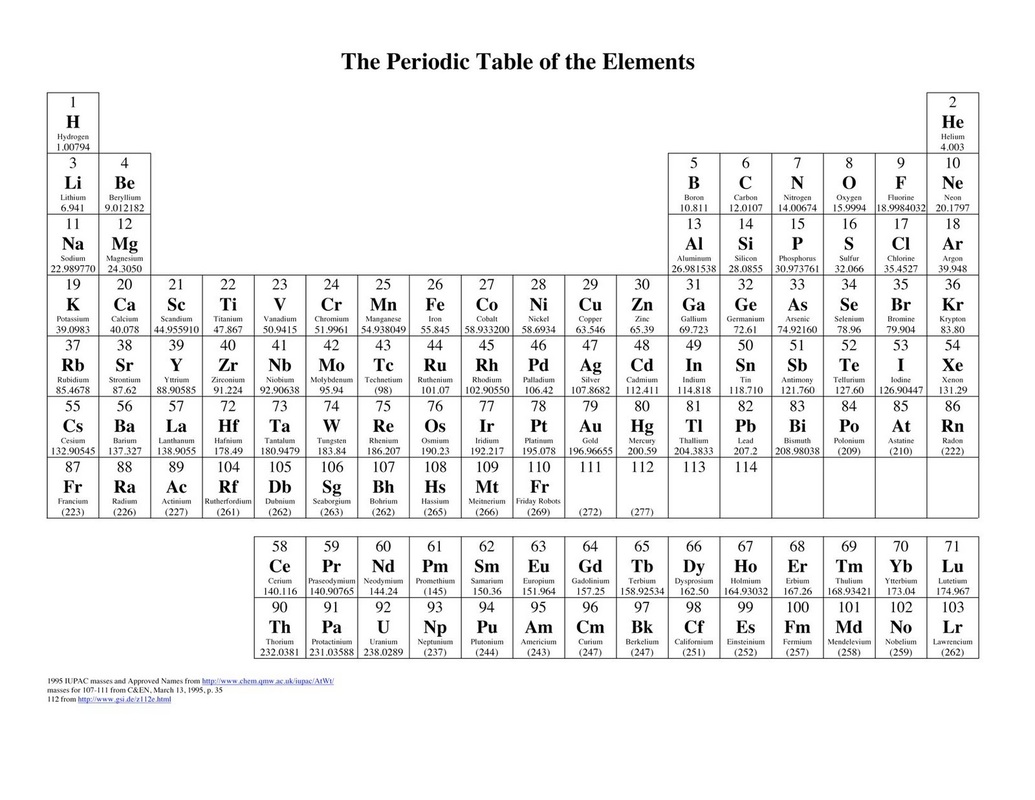
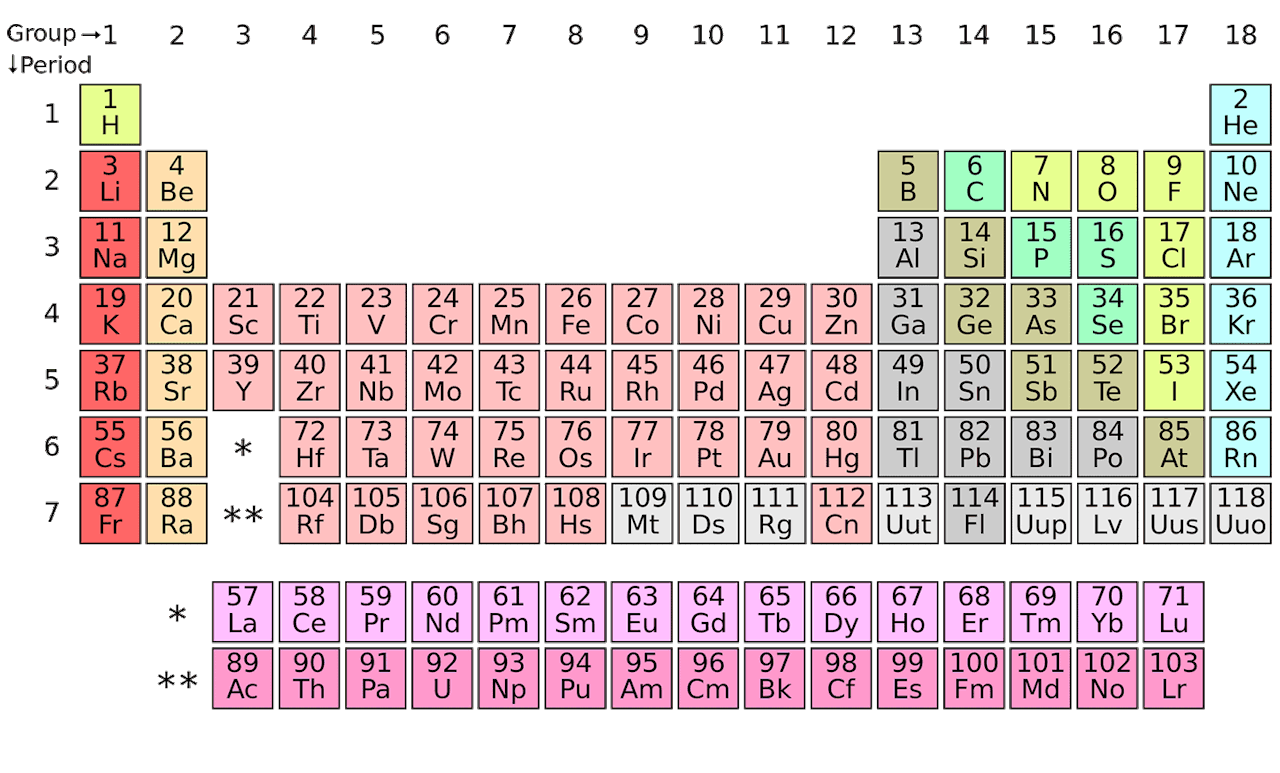
We'll discuss the atom in more depth later in this unit, but it is important to understand how small it is. Electrons, orbiting the nucleus, have a negative charge and counteract the positive center of the atom. Since protons have a + charge and neutrons are neutral, the nucleus is very overall very positive. The nucleus is a small, dense core at the center of the atom. Protons and neutrons are located in the nucleus at the center of the atom, while electrons orbit the nucleus. There are different models of an atom, but the above is an example of where subatomic particles may exist. This activity will help your students to experience the table and conceptualize its trends in a deeper way.Image Courtesy of Let's Talk Science. A deep understanding of the periodic table is the most critical knowledge in chemistry. Science is creative it requires new ideas, new patterns, and new solutions to old problems. The Chemistry of a Family-Style Dinner - Allison Tarvin Students explore trends related to their own topic and relate to the trends on the actual Periodic Table of Elements. Students choose a topic and select items to incorporate into a periodic table of their own design. There are many diagrams and explanations available as resources for students however, a deeper understanding may be possible when students discover these trends independently through a game called Electron Configuration Battleship.Ĭonnecting Black Panther's Vibranium to the Periodic Table - Sibrina CollinsĪ 2018 letter published in the Journal of Chemical Education,"Black Panther, Vibranium and the Periodic Table", describes how the movie, Black Panther, provides a unique opportunity for students to think critically about the arrangement of the periodic table. Many novice students struggle to see elements' valence electron configuration trends across the rows and columns on the periodic table.
This virtual adaptation to a Target Inquiry card sort activity provides students the opportunity to engage in a process similar to the one Mendeleev used as he constructed the original version of the periodic table we still use today. This lesson was designed to fit the NGSS performance expectation HS-PS 1.1 but can be used for any first year chemistry course or modified at your discretion.Ī Virtual Adaptation of a Periodic Table Card Sort and Lab - Chad Husting Students will identify trends that are consistent from one table to the next in order to understand why the tables they are working with and Mendeleev's version are organized in the manner that they are. In this lesson, students are offered a variety of alternative versions of the periodic table. Other ChemEd X content linked to the Periodic Table and Periodic Trends Students often memorize trends, but to get a true grasp of their meaning and what causes certain patterns is best understood when students create their own models and discuss the patterns with others. Trends related to placement of elements on the periodic table are often taught using diagrams in a textbook.
Periodic Trends Guided-Inquiry Activity - Ann Baxley Nora also recommended the following ChemEd X activity:
#Ap chem periodic table free
During the discussion, Nora Walsh shared a free virtual version of the Periodic People activity that she found on the SunriseScience blog. Students manipulate the people within a Google Slide to create the table digitally. If you check out the slide deck or the Resource folder above, you will find a Missing Person activity (aka Periodic People activity). Video of Periodic Trends with Rachmad TjachyadiĬhemEd X ChemBasics Talk Recording: Edited video of Rachmad's ChemBasics Talk - The Periodic Table and Periodic Trends, ChemEd X Vimeo Channel () Access to Shared MaterialsĬhemBasics Periodic Table presentation Slide DeckĪll Resources shared by Rachmad (these were all shared in the Slide Deck above)ĭuring the presentation, Rachmad recommended a Coulombic Attraction activity he uses that is published in POGIL Activities for High School Chemistry.


 0 kommentar(er)
0 kommentar(er)
